How to Find Theoretical and Percent Yield in Chemistry
If you've ever searched "how to find theoretical yield" or wondered about percent yield calculation, you're learning one of the most practical skills in chemistry. Understanding theoretical yield meaning helps you predict how much product a reaction can make, while percent yield tells you how efficient that reaction actually was.
These calculations aren't just for tests — they're how real chemists measure success in the lab and plan industrial production. Whether you're completing homework, preparing for an exam, or working in a laboratory, mastering yield calculations will make chemistry feel much more manageable.
This guide breaks everything down step-by-step with clear formulas, definitions, and a worked example. Let's make yield calculations simple.
“Theoretical yield is the maximum amount of product that can form from given reactants, based on the balanced chemical equation. Percent yield measures reaction efficiency using the formula: Percent Yield = (Actual Yield ÷ Theoretical Yield) × 100.”
What Are Theoretical Yield and Percent Yield in Chemistry?
Before diving into calculations, let's clarify what these terms actually mean. Understanding the definitions first makes the math much easier to follow. According to Khan Academy's chemistry resources, these concepts are fundamental to stoichiometry and reaction analysis.
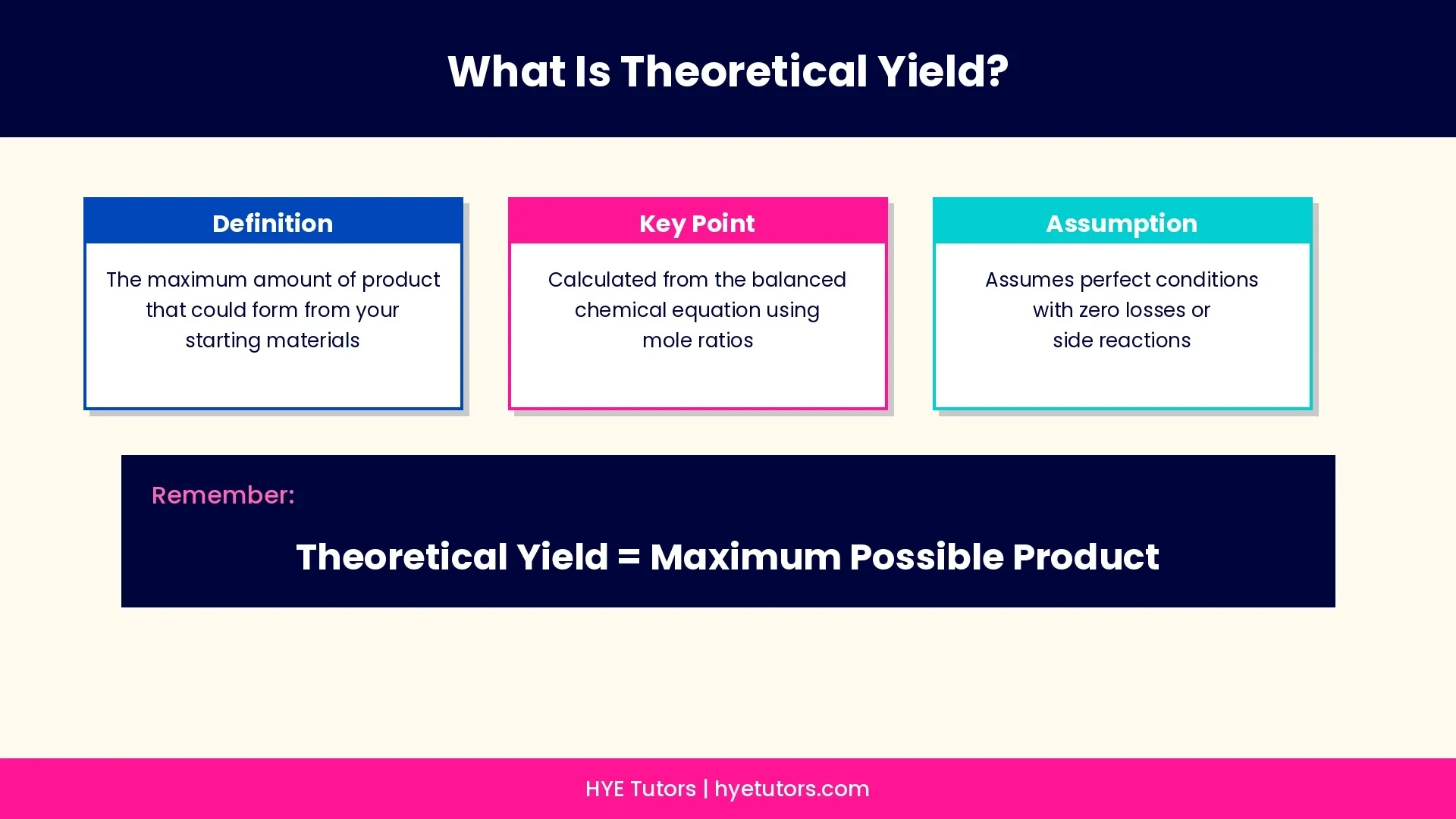
Theoretical Yield:
• The maximum amount of product that could possibly form from your starting materials
• Calculated using mole ratios from the balanced chemical equation
• Assumes perfect conditions with no losses
Actual Yield:
• The real amount of product you actually obtain in the lab
• Always measured experimentally — not calculated
Percent Yield:
• Compares actual yield to theoretical yield
• Measures how efficient your reaction was
• Expressed as a percentage
Why Do Chemists Calculate Yield?
Yield calculations aren't just academic exercises — they have real-world applications that matter in labs, factories, and research facilities every day.
• Measuring reaction success: Determine if a reaction worked as expected
• Reducing waste and cost: Identify inefficiencies and save materials
• Planning industrial production: Calculate how much reactant is needed for desired output
• Reporting lab results accurately: Communicate findings in standardized terms
Key Chemistry Terms You Need Before Calculating Yield
Before calculating yield, make sure you understand these foundational concepts. Getting these basics right prevents common calculation errors.
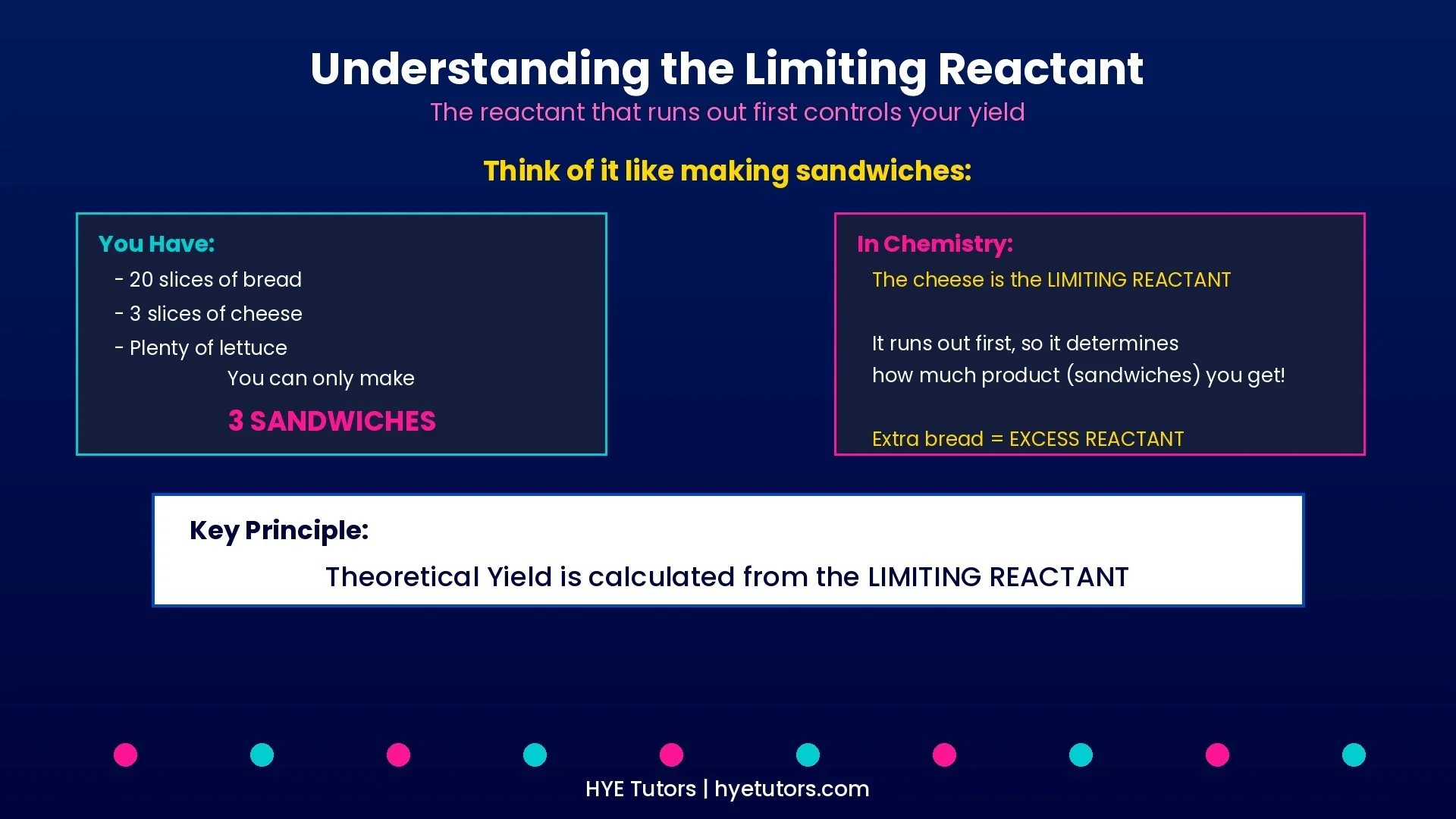
Reactants, Products, and Limiting Reactant
• Reactants: The starting substances that combine in a chemical reaction
• Products: The new substances formed after the reaction
• Limiting reactant: The reactant that runs out first — it controls how much product can form and determines your theoretical yield
Mole Ratios from Balanced Equations
• Coefficients in balanced equations show the ratio between substances
• Used to convert moles of reactant to moles of product
• Essential for calculating theoretical yield accurately
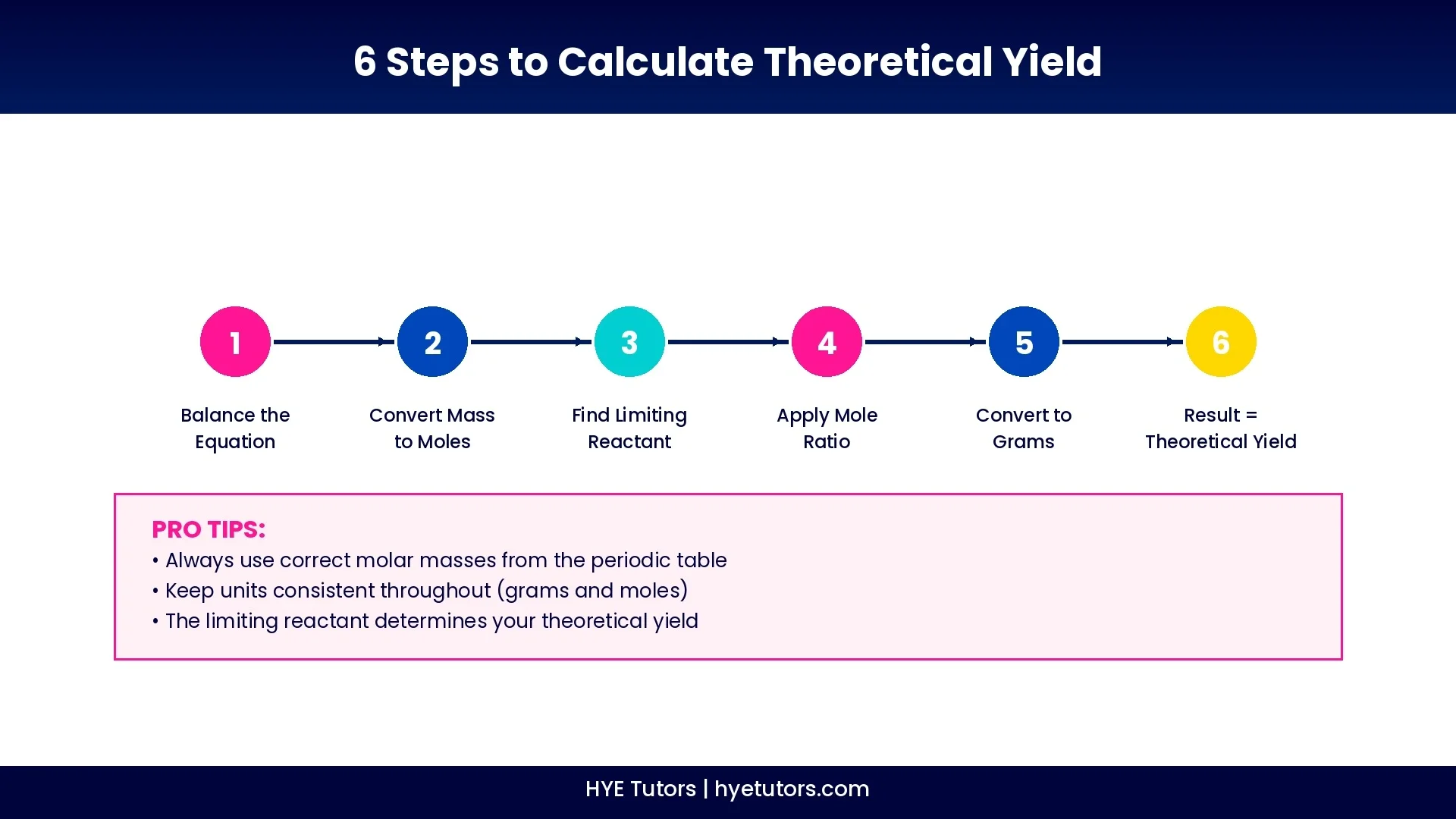
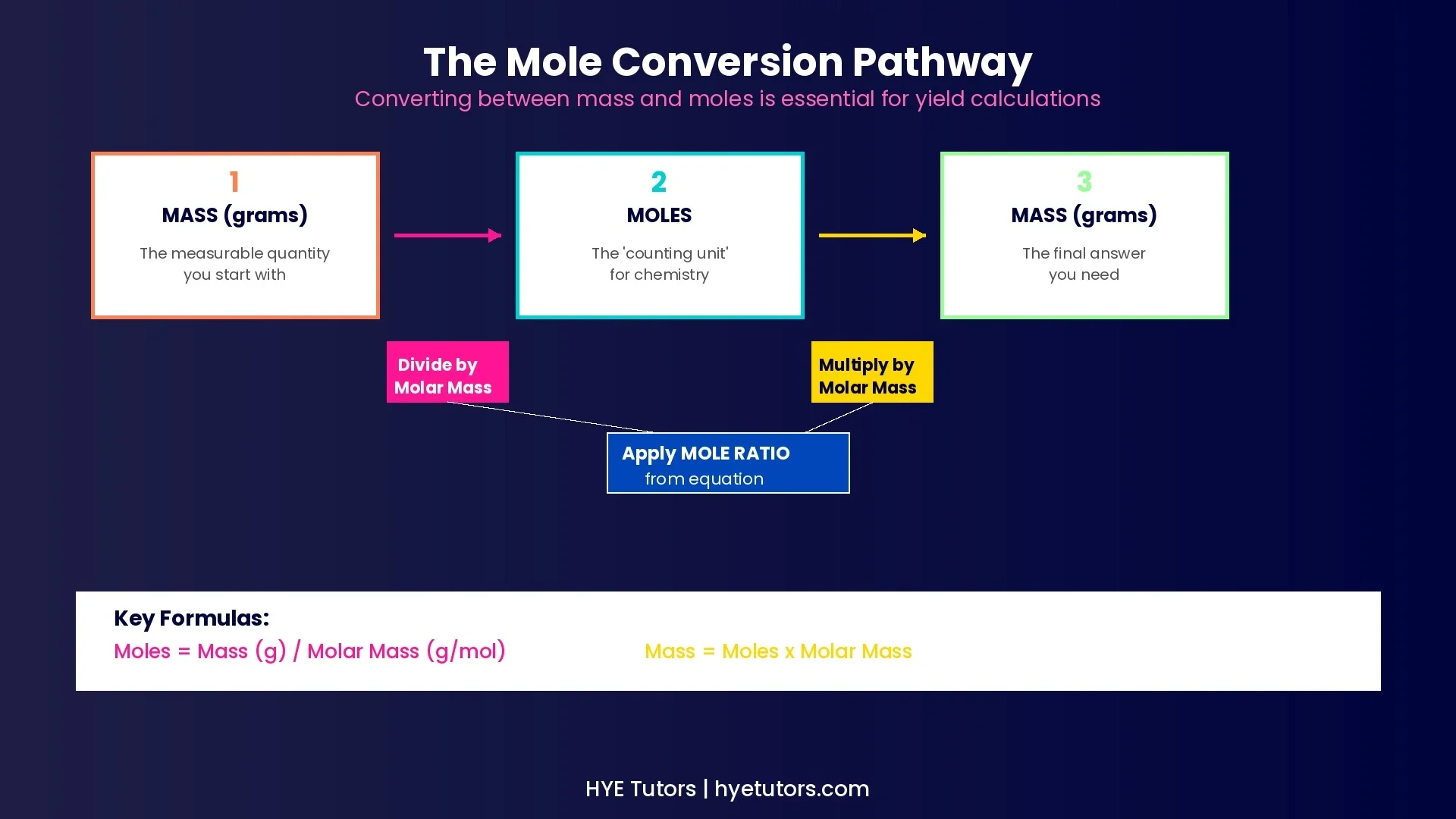
How to Find Theoretical Yield (Step-by-Step Guide)
This is the core calculation students search for most often. Follow these steps carefully, and you'll be able to calculate theoretical yield for any reaction. The American Chemical Society recommends mastering this process as a foundation for all stoichiometry work.
Write and balance the chemical equation. Make sure coefficients accurately represent the reaction.
Convert the reactant mass to moles. Use the formula: moles = mass (g) ÷ molar mass (g/mol).
Identify the limiting reactant. Compare mole ratios to find which reactant runs out first.
Use the mole ratio to find moles of product. Apply the coefficient ratio from your balanced equation.
Convert product moles to grams. Multiply moles by the product's molar mass.
Result = theoretical yield. This is the maximum product possible.
Important reminders: Always use correct molar masses from the periodic table, and make sure your units match throughout the calculation.
How to Find Percent Yield in Chemistry
Once you have both theoretical yield and actual yield, calculating percent yield is straightforward. This formula compares what you actually got to what was theoretically possible.
Percent Yield = (Actual Yield ÷ Theoretical Yield) × 100
• Actual yield: The amount you measured in the lab (given in the problem or experiment)
• Theoretical yield: The amount you calculated using stoichiometry
• Result: Expressed as a percentage
What percent yield tells you:
• 100% = Perfect efficiency (rare in practice)
• Less than 100% = Normal — some product was lost
• Above 100% = Likely a measurement error or impure product
Simple Worked Example (Beginner-Friendly)
Let's work through a complete example using the synthesis of water:
Reaction: 2H₂ + O₂ → 2H₂O
Given: 4 grams of H₂ reacts with excess O₂. Actual yield obtained: 32 grams of H₂O.
Step 1 — Convert H₂ to moles: 4 g ÷ 2 g/mol = 2 moles H₂
Step 2 — Use mole ratio: 2 mol H₂ → 2 mol H₂O (1:1 ratio from equation)
Step 3 — Convert to grams: 2 mol × 18 g/mol = 36 grams H₂O
Theoretical Yield: 36 grams
Percent Yield: (32 ÷ 36) × 100 = 88.9%
Interpretation: The reaction was 88.9% efficient — a typical result for a lab setting.
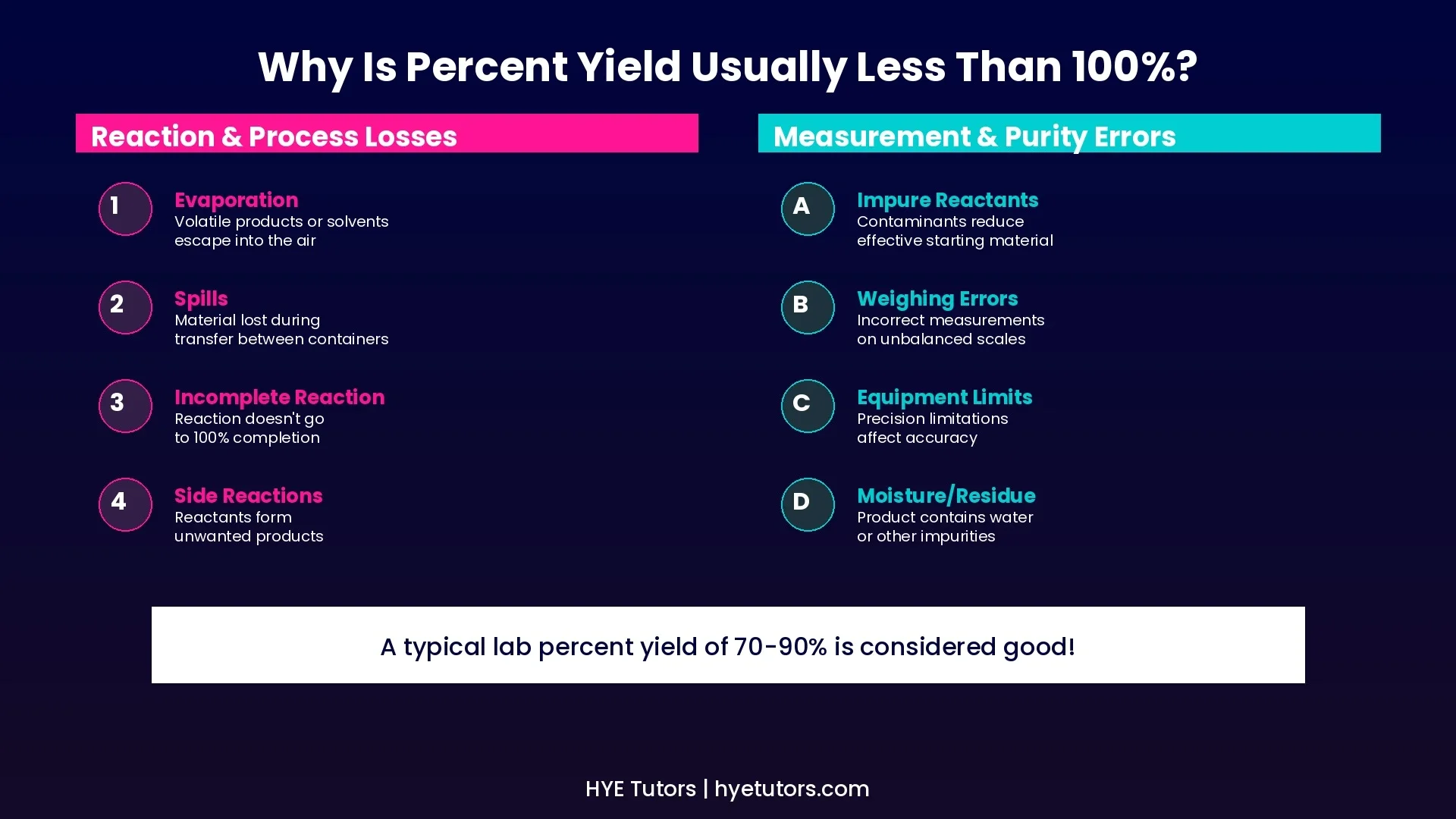
Why Percent Yield Is Usually Less Than 100%
In real chemistry, reactions rarely achieve 100% yield. Understanding why helps you interpret your results and improve your technique.
Reaction & Process Losses
• Evaporation of volatile products or solvents
• Spills during transfer between containers
• Incomplete reactions that don't go to completion
• Side reactions producing unwanted products
Measurement & Purity Errors
• Impure reactants containing contaminants
• Incorrect weighing on unbalanced scales
• Equipment limitations affecting accuracy
Common Mistakes Students Make in Yield Calculations
Avoiding these errors will save you time and improve your accuracy. Think of this as a quick checklist before submitting your work:
• Forgetting to balance the equation: Unbalanced equations give wrong mole ratios
• Ignoring the limiting reactant: Always identify which reactant controls the yield
• Using wrong molar mass: Double-check values from the periodic table
• Mixing units: Keep everything in grams and moles consistently
• Rounding too early: Wait until the final answer to round
Quick Summary — Formulas You Must Remember
Keep these key formulas handy for homework and exams:
Theoretical Yield: Calculate from mole ratios using the limiting reactant → gives maximum possible product
Percent Yield: (Actual Yield ÷ Theoretical Yield) × 100
Remember: Theoretical yield = maximum possible. Percent yield = efficiency of the reaction.
FAQs
What is the difference between theoretical yield and actual yield?
Theoretical yield is the calculated maximum product possible based on stoichiometry. Actual yield is what you physically measure after performing the experiment. Theoretical is always calculated; actual is always measured.
Why is percent yield important in chemistry?
Percent yield measures reaction efficiency, helping chemists evaluate success, reduce waste, plan production quantities, and compare different reaction methods. It's essential for both laboratory work and industrial manufacturing.
Can percent yield be more than 100%?
Technically, no — but it can appear that way due to measurement errors, impure products containing extra mass, or incomplete drying. A percent yield above 100% usually indicates something went wrong with measurement or purification.
Do you always need the limiting reactant to calculate yield?
Yes. The limiting reactant determines how much product can form. If one reactant is in excess, only the limiting reactant's amount matters for calculating theoretical yield.
Is theoretical yield the same as maximum possible product?
Yes. Theoretical yield represents the maximum amount of product that could form if the reaction went perfectly with no losses. It's the ideal outcome based purely on stoichiometric calculations.
Conclusion
Understanding yield calculations is one of the most practical skills you'll learn in chemistry. Theoretical yield tells you the maximum product possible based on your starting materials and balanced equation. Percent yield shows you how well your actual reaction performed compared to that ideal.
Now you know how to find theoretical yield by following the step-by-step process: balance the equation, convert to moles, identify the limiting reactant, apply mole ratios, and convert back to grams. You also know how to find percent yield using the simple formula that compares actual to theoretical results.
With practice, these calculations become second nature. Keep working through examples, check your units carefully, and you'll master yield calculations in no time. You've got this!